China National Medical Products Administration, NMPA, issued Notice No. 32, namely Provisions for Management of Cosmetic Registration and Notification Dossiers, providing detailed requirement on documents and process of Notification and Registration of cosmetic products. Here are the highlights in this new provision.
- Animal test can be replaced by safety assessment for imported general cosmetics if the cosmetic manufacturers receive manufacturing certificate from local authority of where the manufacturers locate. There are three exceptions:
- Products is intended for use on infant or children
- Products contain new raw material
- There is a poor record of responsible person in NMPA
- Uploading of cosmetic formulation with exact weight percentage is required during notification and registration. This includes safety information file of every raw material.
- Raw material safety information file and code shall be provided for every cosmetic ingredient.
- For cosmetic products which will be exported to overseas, they shall be proceeded with notification to NMPA with basic information, including product name, countries for exportation and product label. Manufacturers shall conduct testing according to the requirements of the target markets.
With the new regulations set by the NMPA, special products follow a stricter procedure for notification and registration: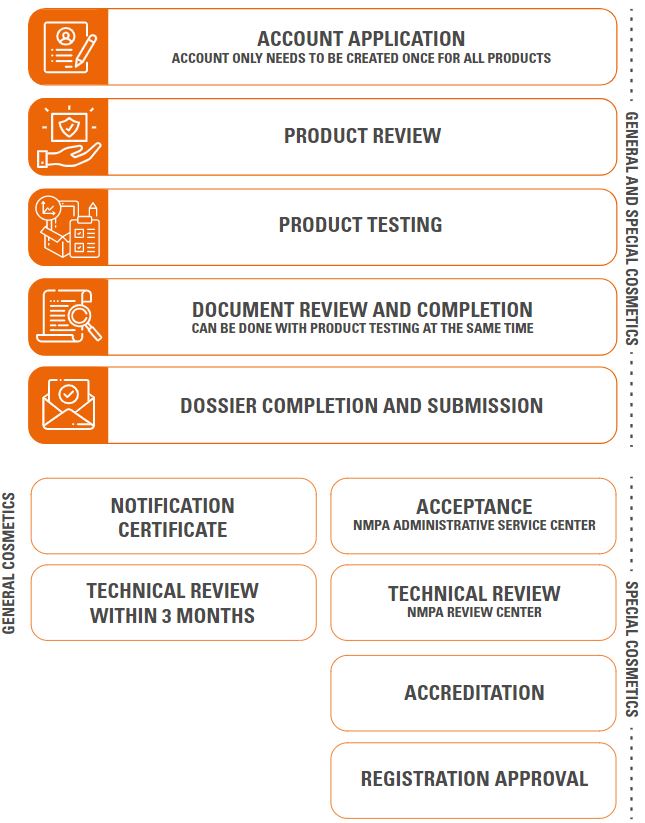
We are SGS – the world’s leading testing, inspection, and certification company. We are recognized as the global benchmark for quality and integrity. Our experts worldwide provide specialized solutions to make your business faster, simpler, and more efficient.
To get to know more about SGS Solution on China Import Services for cosmetics and skincare products, please fill in the below form to receive the brochure and subscribe to our newsletter. You can also contact us or learn more China Import Packages at TIC Mall.
"*" indicates required fields



